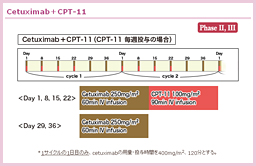
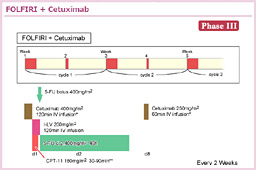
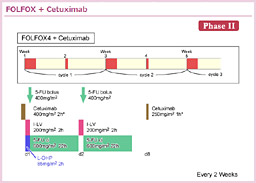
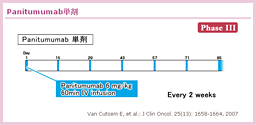
@2000NãÉüèAå°àÌÌæÉààÌBE]ÚÉ©©íéÁè̪qÉWIðiÁ½ªqWI¡ÃªÕ°±ü³êAù¶Ì»wÃ@Æ̹pÉæÁÄA³çÈ鶶úÔÌ·¨æÑtø¦Ìüãª}çê½BܽAgoCI}[J[Éæé³ÒIðhÆ¢¤TOª»ÀÌàÌÆÈèApersonalized therapyÆ¢¤V½ÈãÌJ¯ð}¦½B
@Çàç×EBöqiVEGF: vascular endothelial growth factorjͽÌàíÅ»µÄ¢é±ÆªmçêĨèA³çɻ̻ÍîáÌZâ]ÚAĨæÑ\ãÉÖA·éBBevacizumabiAoX`®jÍAVEGFÉηéL^qg»IgG1mN[iRÌÅ èAîáÇV¶ð}§·é±ÆÉæèAîáÌB é¢Í]Úð}§·é±ÆªúÒ³êéB
@
BevacizumabÉNö·éÁ¥IÈò¨LQ½ÆµÄÍAoi16.7%jAÁ»ÇúEi0.9%jAáEi0.3%jAðÇðÇA³«]Çipxs¾jÈǪmçêÄ¢é44)B
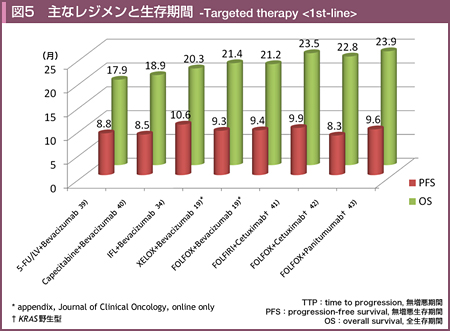
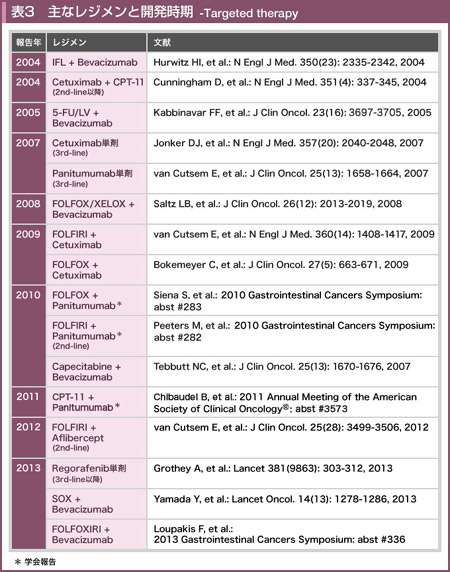
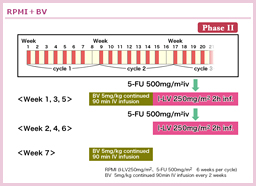
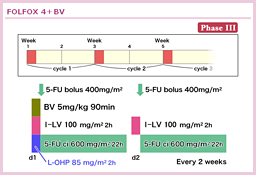
@KabbinavarçÍABevacizumabð¹pµ½3Â̳ì×»är±Ì^AiVXɨ¢ÄA5-FU/LVܽÍIFLÉεA5-FU/LV{BevacizumabÍtø¦i5-FU/LV{Bevacizumab vs. 5-FU/LV or IFL=34.1% vs. 24.5%, p=0.019jAPFSieX8.8 vs. 5.6, HR=0.63, p=0.0001jAOSieX17.9 vs. 14.6, HR=0.74, p=0.008jÌ¢¸êɨ¢ÄàLÓÉãñé±Æ𦵽39)B5-FU/LV{BevacizumabÍACPT-11¨æÑL-OHPsÏáÉηé¡ÃIvVÆF¯³êÄ¢éB
@ñ¡ÃáðÎÛƵÄACapecitabinePÜÆCapecitabine{BevacizumabiCBjACapecitabine{Bevacizumab{Mitomycin Cðärµ½æIII±iMAX±jɨ¢ÄACBQÍCapecitabinePÜQÉεAOSÅÍLÓ·ðFßÈ©Á½àÌÌiCB vs. CapecitabinePÜ=18.9 vs. 18.9, HR=0.88, p=0.31jAåv]¿ÚÅ éPFSieX8.5 vs. 5.7, HR=0.63, p<0.001jÅLÓÉãñèACapecitabineÉηéBevacizumabÌãæ¹øʪ¦³ê½BCapecitabine{BevacizumabÍA5-FU/LV{BevacizumabƯlÉACPT-11¨æÑL-OHPsÏáÉηé¡ÃIvVƵÄF¯³êÄ¢é40)B
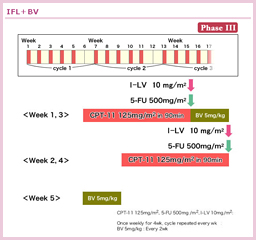
@HurwitzçÍñ¡ÃáðÎÛÉAIFLðRg[A[ƵÄAIFL{BevacizumabÆ5-FU/LV{Bevacizumab̳ì×»är±ðsÁ½iAVF2107g±jBÔðÍɨ¢ÄAIFL{BevacizumabÌÀS«ªEeÂ\Å Á½±ÆæèA{±ÍIFLÆIFL{BevacizumabÌ2QÔÌär±ÆµÄp±³ê½B»ÌÊAåv]¿ÚÅ éOSiIFL{Bevacizumab vs. IFL=20.3 vs. 15.6, HR=0.66, p<0.001j¨æÑPFSieX10.6 vs. 6.2, HR=0.54, p<0.001jAtø¦ieX45% vs. 35%, p=0.004jÌ¢¸êɨ¢ÄàBevacizumab¹pQªãñÁ½BLQÛÍABevacizumab¹pQų̶¦ªLÓɽ©Á½àÌÌA»Ì¼É¢ÄÍLÓÈ·ðFßÈ©Á½34)B
ENO 16966±
i±fUCÉ¢ÄÍ2.2.3 XELOXQÆj
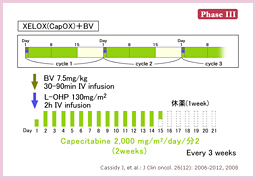
@Saltzçªñµ½XELOX/FOLFOX{BevacizumabÆXELOX/FOLFOXÌärÅÍAtø¦ieX47% vs. 49%, p=0.31j¨æÑOSieX21.3 vs. 19.9, HR=0.89, p=0.077jÉ¢ÄÍLÓ·ðFßÈ©Á½àÌÌAåv]¿ÚÅ éPFSÅÍBevacizumab¹pQªLÓÉãñÁ½ieX9.4 vs. 8.0, HR=0.83, p=0.0023j19)B
@ܽAñ¡ÃƵÄA5-FUÆCPT-11sáðÎÛÉFOLFOX}BevacizumabÌär±ªsíê½Båv]¿ÚÅ éOSÅÍABevacizumab¹pQªLÓÉãñèiFOLFOX{Bevacizumab vs. FOLFOX=12.9 vs. 10.8, HR=0.75, p=0.0011jAPFSieX7.3 vs. 4.7, HR=0.61Ap<0.0001jAtø¦ieX22.7% vs. 8.6%, p<0.0001jɨ¢ÄàBevacizumab¹pQªãñÁ½45)B
@
±êçÌÊæèAê¨æÑñ¡Ãɨ¯éFOLFOX/XELOXÖÌBevacizumab¹pÌLø«ª¦³ê½B
ESOFT±
@SOXÍAoûtb»s~Wn»ÜÅ éS-1ÆL-OHPð¹p·éWÅ éBñ¡Ãɨ¯éW¡ÃÌ1ÂÅ émFOLFOX6{BevacizumabÃ@iFOLFOXQjÉηéSOX{BevacizumabÃ@iSOXQjÌñò«ðص½³ì×»æIII±Å éSOFT±ª{M©çñ³ê½B
@RECISTÅè`³êéPDÍWIaÏÌŬ·aa©ç20%ÈãÌ·aaåÅ éªA{±ÅÍPFSÌCxgƵÄÌPDðAx[XCÌWIaÏÌ·aa©ç20%ÈãÌåÆè`µ½Båv]¿ÚÅ éPFSÌlÍAÏ@úÔl18.4Ì_ÅFOLFOXQ11.5ASOXQ11.7Å èiHR=1.043, 95% CI: 0.860-1.266jAÝèµ½ñò«}[W1.33ðºñÁ½½ßASOXÃ@ÌPFSɨ¯éñò«ª¦³ê½78)BȨA±ÌÊÍRECISTÉîÃ]¿Åà¯lÅ Á½BܽA]¿ÚÅ éOSlàA»ê¼ê30.9A29.6Å èiHR=1.052, 95% CI: 0.805-1.376jA¼QÔÉ·ðFßÈ©Á½Btø¦ÍAFOLFOXQ62.7%ASOXQ61.5%ip=0.8026jA8T_ÅÌtø¦Í»ê¼ê42.9A40.3%ip=0.5669) 79)AÅåîák¬Í»ê¼ê71.7%A72.3%ip=0.8944jAR0ئͻê¼ê8.6%A9.4%ip=0.7678j78)ÆA¼QÔÉ·ðFßÈ©Á½B
@Grade 3ÈãÌåÈLQÛƵÄFOLFOXQÅD
¸ASOXQÅÁ»íÅ«iSAH~sUAºjª½¢XüÉ Á½BܽA{±É¨¢ÄÍFOLFOXQÅ1ái0.4%jASOXQÅ5ái2.0%jÌÁ»ÇúEáðFß½ªA¾ç©È ]ÚðL·éÇáÆxÌÁ»Ç·óðFßéÇáðO·é±ÆÅÈ~̶ðñð·é±ÆªÅ«½B
@Èã©çAñ¡ÃƵÄSOX{BevacizumabÍ¡ÃIðÌ1ÂÅ éÆl¦çêéB
@FuchsçÍAñ¡ÃáðÎÛÉAmodified IFLimIFLj{BevacizumabÆFOLFIRI{Bevacizumabðärµ½æII±iBICC-C± Period 2jðs¢Atø¦iFOLFIRI{Bevacizumab vs. mIFL{Bevacizumab=57.9% vs. 53.3%jAPFSieX11.2 vs. 8.3. p=0.28jɨ¢ÄÍLÓ·ÍFßÈ©Á½àÌÌAOSÍFOLFIRI{Bevacizumabªãñé±ÆieX28.0 vs. 19.2, p=0.037jðñµ½B
@
{ñæèABevacizumabƹp·éÛÌ5-FU/LV{CPT-11WÍFOLFIRIªÅKÅ éÆF¯³êÄ¢é27)B
ETRIBE±
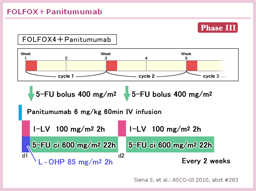
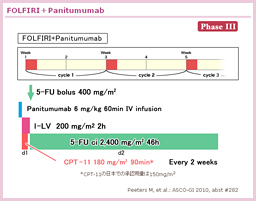
@TRIBE±ÅÍAñ¡Ãɨ¯éFOLFIRI{BevacizumabÆFOLFOXIRI{Bevacizumabªär³ê½BFOLFOXIRIQÌpÊÍACPT-11: 165mg/m2AL-OHP: 85mg/m2ALV: 200mg/m2A5-FU ci: 3,200mg/m2ABevacizumab: 5mg/kgÅ èAFOLFIRIQAFOLFOXIRIQÆàÉÅå12TCN^µ½ãA5-FU/LV{BevacizumabÉæéÛÃ@ðsÁ½B
@Ï@úÔl32.3Ì_ÅAPFSlÍFOLFIRIQ9.7AFOLFOXIRIQ12.1ÆAFOLFOXIRIQÅLÓÈ·ðFß½iHR=0.77, 95% CI: 0.64-0.93, p=0.006j80)BܽAOSlÍ»ê¼ê25.8A31.0ÆAFOLFOXIRIQÅÇDÈXüðFß½iHR=0.83, 95% CI: 0.66-1.05, p=0.125jBâ`qÏÙÊÌPFSÌHRÍAKRAS exon 2ì¶^/ÏÙ^Å0.83/0.84ABRAFì¶^/ÏÙ^Å0.83/0.55ÆAâ`qÏÙÌL³É©©íç¸FOLFOXIRIQÌLø«ª¦´³êAÆèí¯\ãªsÇÈBRAFÏÙ^ɨ¯éLø«ªúÒ³êéÊÅ Á½B
@tø¦ÍFOLFIRIQ53%AFOLFOXIRIQ65%ÆFOLFOXIRIQÅLÓÉÇDÅ Á½ªip=0.006jAÌÀÇ]Úáɨ¯éconversion therapy̨æÑR0ئͼQÔÅLÓ·ðFßÈ©Á½BȨAúîák¬ÆtøÌ[³Éæé¢ÅÍAúîák¬ð¾½ÇáÅÍA¾È©Á½ÇáÆärµÄPFSÌ·ðFßip < 0.0001jAÅåîák¬Ìl (38.9%) ð´¦éîák¬ð¾½ÇáÍAlȺŠÁ½ÇáÆärµÄPFSÌ·ðFß½ip<0.0001j81)B
@åÈgrade 3ÈãÌLQÛÍAD
¸¨æѽ_oáQªFOLFOXIRIQÅLÓɽ©Á½àÌÌiÆàÉp<0.001jAM«D
¸ÇͯöxÅA»Ì¼ÌñtÅ«à¼QÔÅ·ðF߸AEeÂ\Å éÆl¦çê½B
@Èã©çAñ¡ÃÌAÁÉRASÏÙðLµREGFRRÌòÌøʪúÒÅ«È¢Çáɨ¢ÄFOLFOXIRI{BevacizumabÍAOSÌ·ðúÒµ¾éL]ÈWÅ éÆl¦çêéB½¾µOSÌðÍÉ¢ÄÍÏ@úÔª\ªÅÍÈA2nd-lineÈ~Ìe¿ÉæèPFSÌ·ªOSɨ¢ÄÍÛÅ«È¢Â\«ðOªÉ¨Kvª éB
EBRiTEAARIESiÏ@¤j
@Bevacizumab¹p»wÃ@Éæéñ¡ÃÉsÆÈÁ½ãAñ¡ÃÅBevacizumabðp±µÄ¹p·é±Æ (bevacizumab beyond progression: BBP) ÌLp«ÍABRiTE¨æÑARIESÆ¢¤2ÂÌåKÍÏ@¤ÉæÁĦ´³êÄ¢½BBRiTEɨ¯éOSÍBBPQ31.8AñBBPQ19.9ÆABBPQŶ¶úÔªÇDÅA±¢Äñ³ê½ARIESɨ¢ÄàOSÍBBPQ27.5AñBBPQ18.7ÆABRiTEÌÊðx·éÊÅ Á½62, 63)B
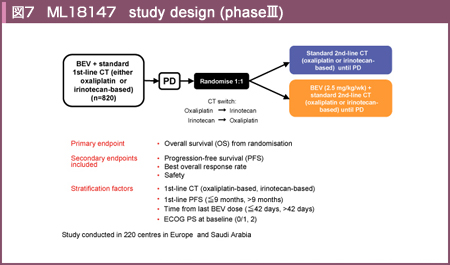
@ML18147±ÍBBPÌLø«ðOü«Éص½³ì×»äræIII±Å éBWIÈBevacizumab¹pÌñ¡ÃsáðÎÛÉAñ¡Ãɨ¯éBevacizumab¹pQiBBPQjÆñ¹pQiñBBPQjð³ì×»ärµ½i}7jBåv]¿ÚÅ éOSÌlÍBBPQ 11.2AñBBPQ9.8iHR=0.81, 95% CI: 0.69-0.94, p=0.0062jÆALÓÉBBPQªÇDÅ Á½64)B]¿ÚÅÍAtø¦ÉLÓ·ðFßÈ©Á½àÌÌi5.4% vs. 3.9%, p=0.3113jAPFSÍBBPQÅLÓÉ·µ½i5.7 vs. 4.1AHR=0.68, 95% CI: 0.59-0.78, p<0.0001jBTuO[vðÍÅÍKRASÏÙÌL³É¢ÄÌîñª¾çê½ÇáðÎÛÉAKRAS statusÊÉBBPÌøʪðͳê½BPFSÍKRASì¶^ɨ¢ÄBBPQ6.4AñBBPQ4.5iHR=0.61, 95% CI: 0.49-0.77, p<0.0001jAKRASÏÙ^ɨ¢ÄBBPQ5.5AñBBPQ4.1iHR=0.70, 95% CI: 0.56-0.89, p=0.0027jÆAKRAS statusÉæç¸BBPÉæéPFSÌ·øʪ¦³ê½ªAOSÍKRASì¶^ÅÍLÓ·ðFß½àÌÌi15.4 vs. 11.1, HR=0.69, p=0.0052)AKRASÏÙ^ɨ¢ÄÍLÓ·ðFßÈ©Á½i10.4 vs. 10.0, HR=0.92, p=0.4969j65)BܽAESMO 2012ÅÍNîÊÌðͪ\³êAOSÍ65΢ɨ¢ÄBBPQ11.6AñBBPQ9.9iHR=0.79, 95% CI: 0.65-0.98, p=0.0274jA65ÎÈãÅÍBBPQ10.7AñBBPQ9.8iHR=0.83, 95% CI: 0.66-1.04, p=0.1056jÆANîÉæç¸BBPQÅÇDÅ Á½66)B
@ÈãÌÊæèABBPÍWIÈ¡ÃíªÌ1ÂÆl¦çêANCCNKChC i2013NÅAversion1jÉàfÚ³êéæ¤ÉÈÁ½67)B½¾µAML18147±ÌÎÛÍñ¡ÃÌPFSª3ÈãÅ èAñ¡Ãɨ¯éBevacizumabªA±µÄ3ñÈã^³êA©ÂBevacizumabÅI^ã3ÈàÉ«µ½ÇáªÎÛÆÈÁÄ¢é±ÆðOªÉuKvª éB
@2012NÌESMOÅÍC^AæèML18147±Éµ½fUC̳ì×»äræII± iBEBYP±jÌʪñ³ê½B±Ì±ÍML18147±Ìö\¨æÑÇáWÏÌxê©ç±ªr~ÆÈÁ½ªAåv]¿ÚÅ éPFSÍBBPQ6.77AñBBPQ4.97iHR=0.65, 95% CI: 0.48-0.89, p=0.0062jÆALÓÉBBPQªÇDÅ Á½68)BȨA]¿ÚÌtø¦ÍBBPQ21%AñBBPQ18%ip=0.71jƼQÅ·ðF߸AOSÍ\ªÈCxgÉBµÄ¢È¢½ßö\³êÈ©Á½B
@ãç×EBöqóeÌiEGFR: Epidermal growth factor receptorjÍAãç×EÈÇÉ»·éÑÊ^ÌóeÌÅ èAKhª·éÆEGFR`VLi[[ª«»³êA×EàVOi`BðîµÄ×EB⪻ª£i³êéBCetuximabiA[r^bNX®jÍAEGFRÉηéIgG1TuNXÌqg/}EXL^ÌmN[iRÌÅAEGFRÉ·é±ÆÉæèA×EBâ]ÚÌ}§ªúÒ³êéB
@
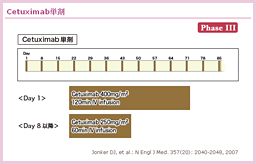
CetuximabÉæéò¨LQ½ÆµÄAdxÌinfusion reactioni<5%jAdxÌçáQi10`15%jAÔ¿«x¾³i<0.5%jAá}OlVEÇAºÈǪñ³êÄ¢é46)B
EFREGFRRÌòÌRîáøÊÆEGFR»
@AREGFRRÌòÍ»Ììp@æèAÆugD»wõFiIHCjÅEGFRð»µÄ¢éå°àðÎÛÉJ³êÄ«½ªA»ÌãÌðÍÉæèAIHCÉæéEGFR»ÌöxiEGFR×EõFÌAEGFR×EõFxjÆREGFRRÌòÌRîáøÊÉ;ç©ÈÖðFßÈ¢ÆÌñ47, 48)ªÈ³êAIHCÉæéEGFR»ÌL³ÍREGFRRÌðgp·éÛÉl¶µÈÄæ¢Æl¦çêéæ¤ÉÈÁÄ¢éB
EFREGFRRÌòÌRîáøÊÆKRASâ`q
@EGFR×EàVOi`BoHÌ1ÂÅ éKRAS/RAF/MAPK`BoHɨ¢ÄAKRASâ`qÉ_ËRÏÙªN±éƺ¬ÖÌPíIÈVOi`BªN±èAEGFRjQÉæéRîáøʪ³øÉÈéÆl¦çêÄ¢éBREGFRRÌòðp¢½½ÌÕ°±ÌðÍ©çà¯lÌñªsíêA»ÝAKRASâ`qÏÙðL·éÇáÉηéREGFRRÌòÌ^ͧ³êĢȢB
@NCI CTG CO.17±ÅÍAtb»s~W¨æÑCPT-11AL-OHPÉsÆÈÁ½ÇáÉεABSCÆÌärÅåv]¿ÚÅ éOSiCetuximab vs. BSC=6.1 vs. 4.6, HR=0.77, p=0.005jA¨æÑPFSieX1.9 vs. 1.8, HR=0.68, p<0.001jAtø¦ieX8% vs. 0%, p<0.001jɨ¢ÄLÓÉãñèAå°àɨ¯éCetuximabPÜ^ÌLø«ª¦³ê½49)B
@»ÌãAKRASâ`qÏÙL³ÊÌgXyNeBuÈðͪsíêAKRASì¶^ÅÍOSÍeX9.5 vs. 4.8iHR=0.55, p<0.001jAPFSÍeX3.7 vs. 1.9iHR=0.40, p<0.001jAtø¦Í12.8% vs. 0%ÆLø«ª¦³ê½BêûAKRASâ`qÏÙ^ÅÍOSÍeX4.5 vs. 4.6iHR=0.98, p=0.89jAPFSÍeX1.8 vs. 1.8iHR=0.99, p=0.96jAtø¦Í1.2% vs. 0%ÆLø«ðFßÈ©Á½50)B
@
LQÛÉ¢ÄÍAç]Aá}OlVEÇÌpxªCetuximabQÅLÓɽ©Á½49)B
EEPIC±
@EPIC±ÅÍA5-FUAL-OHPsáÉηéCPT-11ÖÌCetuximabÌãæ¹øʪ¢³ê½B»ÌÊAPFSÅÍCPT-11{CetuximabQªLÓÉãñÁ½àÌÌiCPT-11{Cetuximab vs. CPT-11=4.0 vs. 2.6, HR=0.69, p≤0.0001jAåv]¿ÚÅ éOSÉ¢Äͼ¡ÃQÔÅ·ªÂ©È©Á½ieX10.7 vs. 10.0, HR=0.98, p=0.71jB±êÍACPT-11PÆQÌñ47%Åã¡ÃƵÄCetuximabªgp³ê½±ÆÉæéÆl¦çêÄ¢é51)B
@{ÊæèA¶¶úÔÌÏ_©çÍCetuximaḇüúÍñ¡ÃAO¡ÃÌ¢¸êÅàæ¢Æl¦çêÄ¢éB
EBOND±
@BOND±ÅÍACPT-11sáÉηéCPT-11{CetuximabÆCetuximabPܪär³ê½BOSÅÍLÓ·ðFßÈ¢àÌÌACPT-11¹pQÅÇDÈXüðFßiCPT-11{Cetuximab vs. CPT-11=8.6 vs. 6.9, HR=0.91, p=0.48jAåv]¿ÚÅ étø¦ieX23% vs. 11%, p=0.007j¨æÑTTPieX4.1 vs. 1.5, HR=0.54, p<0.001jɨ¢ÄCPT-11¹pQªLÓÉãñÁ½B»ÌÊACPT-11sáÉεÄàCPT-11{CetuximabªCetuximabPÜæèàLøÅ é±Æª¦³ê52)ACPT-11sÏáÅȯêÎCPT-11ð¹p·é±Æª§³êÄ¢éB
ECRYSTAL±
@CRYSTAL±ÅÍAñ¡ÃðÎÛÉFOLFIRIÉηéCetuximabÌãæ¹øʪ¢³ê½BKRASâ`qÏÙáðÜÞSÌÅÍAOSɨ¢ÄÍLÓ·ªÈ©Á½àÌÌiFOLFIRI{Cetuximab vs. FOLFIRI=19.9 vs. 18.6, HR=0.93, p=0.31jAåv]¿ÚÅ éPFSÍACetuximab¹pQªLÓÉÇDÅ Á½ieX8.9 vs. 8.0, HR=0.85, p=0.048jB
@»ÌãAKRASâ`qÏÙL³ÊÌgXyNeBuÈðͪsíêAKRASì¶^ÅÍPFSieX9.9 vs. 8.7, HR=0.68, p=0.02jAOSieX24.9 vs. 21.0, HR=0.84jAtø¦ieX59% vs. 43%, IbYä1.91jÌ·×ÄÌRîáøÊÅLÓÉCetuximab¹pQªãñÁ½ªAKRASâ`qÏÙ^ÅÍPFSieX7.6 vs. 8.1, HR=1.07, p=0.75jAOSieX17.5 vs. 17.7, HR=1.03jAtø¦ieX36% vs. 40%, IbYä0.80jÆCetuximabÌãæ¹øÊͦ¹È©Á½53)B±ÌÊæèAKRASâ`qì¶^Ìñ¡ÃáÉηéCetuximabÌãæ¹øʪ¦³ê½B
EFIRE-3±
@FIRE-3±ÅÍAñ»wÃ@ɨ¢ÄAFOLFIRIÃ@ðx[XƵÄCetuximabÆBevacizumabª¼Úär³ê½B±JnÉÍKRAS statusÉæç¸o^ªsí꽪AãÉKRAS exon 2ì¶^ÉÀèµÄo^³êAåðÍÌÎÛÆÈÁÄ¢éB
@åv]¿ÚÅ étø¦ÍACetuximabQ62.0%ABevacizumabQ58.0%ÅA¼QÔÉLÓ·ðFßÈ©Á½iodds ratio=1.18, 95% CI: 0.85-1.64, p=0.183j82)BܽAPFSlÍ»ê¼ê10.0A10.3ƯöxÅ Á½àÌÌiHR=1.06, 95% CI: 0.88-1.26, p=0.547jAOSlÍ»ê¼ê28.7A25.0ÆACetuximabQÅLÓÈ·ðFß½iHR=0.77, 95% CI: 0.62-0.96, p=0.017jBȨAKRAS exon 2ÈOÌRASâ`qÏÙA·Èí¿KRAS exon 3, 4ANRAS exon 2, 3, 4SÄÉÏÙðL³È¢ÇáiRASì¶^jðÎÛÆ·éÆAOSlÍCetuximabQ33.1ABevacizumabQ25.6ÆA¼QÌ·Íæèå«ÈÁ½iHR=0.70, 95% CI: 0.53-0.92, p=0.011j83)BtÉAKRAS exon 2ì¶^©ÂRASÏÙ^ɨ¢ÄÍACetuximabQÅPFSªLÓÉZiHR=2.22, p=0.004jAOSàZ¢XüªÝçê½iHR=1.20, p=0.57jB
@Grade 3ÈãÌåÈLQÛƵÄACetuximabQÅçáQiè«ÇóQA´álç]jâá}OlVEÇAinfusion reactionðæè½Fß½àÌÌ82)Ae³êéÍÍàÆl¦çê½B¼ÌLQÛÉ¢ÄͼQÅ·ðFßÈ©Á½B
@tø¦âPFSÅ·ªÂ©¸AOSÅÌÝLÓ·ªÂ¢½R;mÅÍÈ¢ªAPEAK±ÌÊÆí¹ARASì¶^Ìñ¡Ãɨ¢ÄREGFRRÌò¹pÃ@ªBevacizumab¹pÃ@æèàOSÌ·Éñ^·éÂ\«ª¦´³ê½B½¾µAOSÍ{±Ìåv]¿ÚÅÍÈ¢±ÆABevacizumab¹pQÅBBPibevacizumab beyond progressionjªsíê½ÇáªÈ¢±ÆÈÇA»_ÅREGFRRÌòÌDz«ª¾mÅ éÆ;¢ØêÈ¢B¡ãAñ³êé\è̯lÌRZvgÅ éCALGB80405±ÌÊð¥Ü¦Ac_ðs¤×«Å ë¤BܽPEAK±APRIME±ÌÇÁðÍÊÆí¹AREGFRRÌòÌgpÍRASì¶^Éié׫Æl¦çêéªA{Mɨ¢ÄOqÌRAS¸ÍÛ¯KOÅ èi2013N11»ÝjAWIðÌáÇÆÈÁÄ¢éB
EOPUS±
@OPUS±iæII±jÅÍAñ¡ÃáÉηéFOLFOXPÆÆCetuximab¹pªär³ê½BKRASì¶^ÅÍAOSɨ¢ÄÍLÓ·ðFßÈ©Á½àÌÌiFOLFOX{Cetuximab vs. FOLFOX=22.8 vs. 18.5, HR=0.855, p=0.385jAåv]¿ÚÅ étø¦ieX57.3% vs. 34.0%, p=0.0027j¨æÑPFSieX8.3 vs. 7.2, HR=0.567, p=0.0064jɨ¢ÄCetuximab¹pQªLÓÉãñÁ½B
@
êûAKRASÏÙ^ÅÍtø¦ieX34% vs. 53%, p=0.029jAPFSieX 5.5 vs. 8.6, HR=1.72, p=0.0153jAOSieX13.4 vs. 17.5, HR=1.29, p=0.2004jÆCetuximab¹pQªºñéXüÅ Á½42)B
ECOIN±
@ñ¡Ãɨ¯éFOLFOX / XELOXÉηéCetuximabÌOSɨ¯éãæ¹ðص½±ªCOIN±Å éBKRASì¶^ɨ¢ÄAtø¦ÍCetuximab¹pQ64%A»wÃ@PÆQ57%ip=0.049jÆCetuximab¹pÉæéãæ¹ðFß½àÌÌAåv]¿ÚÅ éOSÍeX17.0 vs. 17.9iHR=1.04, 95% CI: 0.87-1.23, p=0.67jÆACetuximab¹pÉæéDz«ð¦·±ÆªÅ«È©Á½BܽPFSɨ¢ÄàeX8.6 vs. 8.6iHR=0.96, 95% CI: 0.82-1.12, p=0.60jÆLÓ·ðFßÈ©Á½69)BȨATuðÍÅÍKRASì¶^ÌFOLFOX¹p©Â]Ú1ȺÌÇáɨ¢ÄÌÝACetuximabÌãæ¹øʪFßçê½iHR=0.55, 95% CI: 0.35-0.87jB
@NORDIC VII±ÍAk¢É¨¢Ägp³êÄ¢éFLOXWðx[XƵÄAñ¡Ãɨ¢ÄFLOX{CetuximabAFLOXÔ^{CetuximabÛÃ@AFLOXPÆÌ3Q³ì×»är±ÆµÄsíê½Båv]¿ÚÅ éPFSÍAeX8.3 vs. 7.3 vs. 7.9Å èAFLOXPÆQÆFLOX{CetuximabQÆÌÔÉLÓ·ÍFßçêÈ©Á½iHR=0.89, p=0.31j70)BKRASì¶^¨æÑKRASì¶^©ÂBRAFì¶^ÌÇáɨ¢ÄàACetuximab¹pÉæéPFS·øÊÍFßçêÈ©Á½BêûAKRASÏÙ^ɨ¯éPFSÍAFLOX{CetuximabQ9.2AFLOXQ7.8iHR=0.71, p=0.07jÆAFLOX{CetuximabQÅÇDÈXüªÝçê½BܽAOSɨ¢ÄàAKRASì¶^AKRASì¶^©ÂBRAFì¶^AKRASÏÙ^ÌeO[vÅ¡ÃWÉæé·ðFßÈ©Á½B
@OPUS±Ìʪñ³ê½ãANCCNKChCÅÍKRASì¶^ɨ¯éñ¡ÃÌ1ÂƵÄFOLFOX{CetuximabªfÚ³êÄ¢½ªA»ÌãÌ2ÂÌæIII±iCOINANORDIC VIIjÅtb»s~Wn»Ü{L-OHP¹pÃ@ÉηéCetuximabÌãæ¹øÊ𦷱ƪūȩÁ½½ßANCCNKChCÌ2011Nversion 3.2012È~AFOLFOX{CetuximabÍí³ê½B
@±êç2ÂÌæIII±ÅCetuximabÌLp«ð¦¹È©Á½´ö;ç©ÅÍÈ¢ªACOIN±É¨¯éPFSÉÖ·éTuðÍɨ¢ÄOPUS±Æ¯lÉFOLFOXÉεÄÍCetuximabÌãæ¹øÊðFßÄ¢é±Ææè69)AXELOXɨ¯éCapecitabineâFLOXɨ¯é5-FU/LV}¬ÃÈÇA¹p·étb»s~Wn»ÜÆÌÝìpÉâèª Á½Â\«àwE³êÄ¢éB
@PanitumumabixNeBrbNX®jÍEGFRÉηéqg^IgG2mN[iRÌÅ èACetuximab¯lEGFRÉ·é±ÆÉæèAîáB¨æÑ]Ú}§ªúÒ³êéBCetuximabªL^ÌRÌÅ èAinfusion reaction\h̽ßÉRqX^~òÌO^ðv·éÌÉεAPanitumumabÍ®Sqg^RÌÅ é±Æ©çinfusion reactionÌpxªÈiGrade 3Èã <1%j54)AOòÌKèÍÈ¢BܽAòÔuÍCetuximabªT^Å éÌÉεAPanitumumabÍuT^Å éB
@ò¨LQ½ÆµÄAinfusion reactionÌÙ©AçáQAÔ¿«x¾³AºÈÇACetuximabÆT˯lÌñªÈ³êÄ¢éB
E20020408±
@5-FUACPT-11AL-OHPÉsÆÈÁ½ÇáÉεABSCÆÌär±i20020408±jªsíêAåv]¿ÚÅ éPFSÌ·iPanitumumab vs. BSC=8T vs. 7.3T, HR=0.54, p<0.0001jðFßAtø¦É¨¢ÄàLÓÉãñÁ½ieX10% vs. 0%Ap<0.0001jBOSͼQÔÅ·ªÈ©Á½ªiHR=1.00, p=0.81jABSCQÌ76%̳Ҫ{±ÆÀsµÄsíêÄ¢½PanitumumabPÜ^ÌæII±Éo^³êAPanitumumabªgp³ê½ÊÆl¦çêÄ¢é55)B
@
»ÌãKRASÏÙÌL³ÉæéðͪȳêABSCÆÌärɨ¢ÄKRASì¶^ÅÍtø¦17%APFSiPanitumumab vs. BSC=12.3T vs. 7.3T, HR=0.45, p<0.0001jÅãñÁ½ªAKRASÏÙ^ÅÍPFSiPanitumumab vs. BSC=7.4T vs. 7.3T, HR=0.99jÆBSCƯöxÅAtøáÍFßÈ©Á½56)B
EASPECCT±
@20020408±ÅÍBSCQÌ76%ÅPanitumumabª^³ê½±Æ©çOSÉLÓ·ðFßÈ©Á½½ßANCI CTG CO.17±ÅOSÉLÓ·ðFß½CetuximabÉεÄPanitumumabÌñò«ðØ·éæIII±AASPECCT±ªsíê½B
@tb»s~WAL-OHPACPT-11ÉsÅA©ÂREGFRRÌòÌgpðÌÈ¢KRAS exon 2ì¶^ÌÇáðÎÛƵAåv]¿ÚÅ éOSlÍACetuximabQ10.0APanitumumabQ10.4ÅiHR=0.97, 95% CI: 0.84-1.11, ñò«èp=0.0007, retention rate=1.06jACetuximabÉηéPanitumumabQÌñò«ª¦³ê½84)BܽAPFSlÍ»ê¼ê4.1A4.4iHR=1.002, 95% CI: 0.882-1.138jAtø¦Í»ê¼ê22.0%A19.8%Å èA¼QÔÉ·ðFßÈ©Á½BȨAã¡ÃÌ{s¦â»Ìàeà޵Ģ½B
@åÈLQÛͼQÅT˯öxÅ Á½ªAá}OlVEÇÍPanitumumabQ28.8%ACetuximabQ18.9%ÆPanitumumabQŽAinfusion reactionÍ»ê¼ê2.8%A12.5%ÆCetuximabQŽ¢XüÉ Á½B
@ÈãæèAtb»s~WAL-OHPACPT-11Éæé¡ÃðÌ éKRAS exon 2ì¶^ɨ¯éPanitumumabPÜÍACetuximabPÜƯöxÌRîáøÊðLµAÅ«vt@Cà޵Ģ½B
EPRIME±
@PRIME±ÅÍAñ¡Ãáɨ¯éFOLFOXÉηéPanitumumabÌãæ¹øʪ¢³ê½B
@
KRASì¶^ɨ¢ÄAtø¦iFOLFOX{Panitumumab vs. FOLFOX=55% vs. 48%, p=0.07jAOSieX23.9 vs. 19.7, HR=0.83, p=0.07jÅÍLÓ·ðFßÈ©Á½àÌÌPanitumumab¹pQÅÇDÈXüÉ èAåv]¿ÚÅ éPFSÍAPanitumumab¹pQÅLÓÉFOLFOXPÆQðãñÁ½ieX 9.6 vs. 8.0, HR=0.80, p=0.02jB
@êûAKRASÏÙ^ÅÍPFSieX7.3 vs. 8.8, HR=1.29, p=0.02jAOSieX15.5 vs. 19.3, HR=1.24, p=0.07jAtø¦ieX40% vs. 40%, p=0.98jÆAPanitumumab¹pQªºñéXüÉ Á½43)B±ÌÊ©çAKRASì¶^Ìê¡Ãɨ¯éPanitumumabÌLø«ª¦³ê½B
@ȨA2011NÄÕ°îáwïNWïÉÄPRIME±ÌÅIñƵÄo^I¹30ãɨ¯éðÍʪñ³ê½BPFSÍFOLFOX{PanitumumabQ10.0AFOLFOXQ8.6iHR=0.80, 95% CI: 0.67-0.95, p=0.01jAtø¦ÍeX57% vs. 48%ip=0.02jÆPanitumumab¹pQªLÓÉÇDÅ Á½ªAOSÉ¢ÄÍññƯlAeX23.9 vs. 19.7iHR=0.88, 95% CI: 0.73-1.06, p=0.17jÆAPanitumumab¹pQÅÇDÈXüÅ éàÌÌLÓ·ÍFßÈ©Á½71)BܽAKRASÏÙ^ɨ¢ÄàññƯlAPanitumumab¹pQÅPFSÍÆàɺñéXüÉ Á½iHR=1.27, 95% CI: 1.04-1.55, p=0.02jAOSàZ¢XüÉ Á½iHR=1.17, 95% CI: 0.95-1.45, p=0.15jB
@2013NÄÕ°îáwïNWïÅÍATõIðÍƵÄCxg¶¦82%_ɨ¯éOSðͪñ³ê½BKRAS exon 2ì¶^ɨ¯éOSlÍFOLFOX4Q19.4APanitumumab¹pQ23.8Å èAPanitumumab¹pQªLÓÉãñÁ½iHR=0.83, 95% CI: 0.70-0.98, p=0.027j85)BêûKRAS exon 2ÏÙ^ɨ¢ÄÍOñÌñƯlAPanitumumab¹pQªºñéXüÉ Á½iHR=1.16, 95% CI: 0.94-1.41, p=0.162jB
@ܽAOÉvæ³êÄ¢½TõIoCI}[J[¤ÆµÄAKRAS exon 3, 4ANRAS exon 2, 3, 4ÏÙÌL³ÉæéRASðͪñ³êAÅVÌOSðÍ_ɨ¯éRASðÍÌÊàESMO 2013Åñ³ê½B±êçRASâ`qÌÏÙÍ90%ÌÇáÅðÍÂ\Å èAKRAS exon 2ì¶^̤¿17%ɼ̢¸ê©ÌRASÏÙðFß½86)BSÄÌRASÉÏÙðFßÈ¢RASì¶^ɨ¯éPFSÌHR=0.72iKRAS exon 2ì¶^ÌHR=0.80jAOSÌHR=0.77iKRAS exon 2ì¶^ÌHR=0.83j87)Å èARASì¶^ÅPanitumumab¹pÌøʪæ袱ƪ¦³ê½BêûA¢¸ê©ÌRASÏÙðL·é³ÒQɨ¢ÄÍAPFSÌHR=1.31ip=0.008j86)AOSÌHR=1.21ip=0.040j87)ÅALÓÉPanitumumab¹pQªòÁÄ¢½B
@±ÌRASðÍÉæé¢É¨¢ÄAPanitumumabÍRASì«^ÅÌÝ»ÌøÊðóÅ«é±Æª¦³ê½BPanitumumabÌgpɨ¢ÄÍSÄÌRAS¸ªßçêéÆl¦çêéB
EPEAK±
@PEAK±ÍAKRAS exon 2ì¶^Ìñ¡Ãáɨ¢ÄAFOLFOX{PanitumumabÆFOLFOX{Bevacizumabðärµ½³ì×»æII±Å éBññÅÍPanitumumabQ142áABevacizumabQ143áªITTðÍÌÎÛÅ èAåv]¿ÚÅ éPFSÍPanitumumabQ10.9ABevacizumabQ10.1ÅA¼QÔÉLÓ·ðFßÈ©Á½iHR=0.87, 95% CI: 0.65-1.17, p=0.35j88)BܽAtø¦Í»ê¼ê58%A54%ALQÛÉæé¡Ã~Í»ê¼ê24%A27%ÆA¢¸êà¼QůŠÁ½BêûOSÍAPanitumumabQÅÍl¢BABevacizumabQ25.4Å èALÓ·ÍÈ¢àÌÌPanitumumabQÅÇDÈXüÅ Á½iHR=0.72, 95% CI: 0.47-1.11, p=0.14jB
@»ÌãAOÉvæ³êÄ¢½KRAS exon 3, 4ANRAS exon 2, 3, 4ABRAF exon 15ÉæéÇÁðͨæÑOSÌupdateªñ³ê½BITTðÍWcÌ80%Åâ`qðͪsíêAPanitumumabQ88áABevacizumabQ82áªAKRASANRASÌ¢¸êÌÏÙàL³È¢RASì¶^ƵÄÇÁðÍÎÛÆÈÁ½BRASì¶^ɨ¯éPFSlÍAPanitumumabQ13.0ABevacizumabQ10.1ÅAPanitumumabQÅLÓÈ·ðFß½iHR=0.66, 95% CI: 0.46-0.95, p=0.03j89)BܽAOSlà»ê¼ê41.3A28.9ÆAPanitumumabQÅÇDÈXüÅ Á½iHR=0.63, 95% CI: 0.39-1.02, p=0.06jBȨA2nd-lineɨ¯éPanitumumabQÌRVEGF»ÜÉæé¡Ã¦ABevacizumabQÌREGFRRÌòÉæé¡Ã¦ÍAÆàÉ40%öxÅ Á½B
@ÈãA{±ÍO̼àÝèªÈ©Á½±ÆªwE³êéàÌÌAñ¡ÃƵÄÌFOLFOX{PanitumumabÍAÎÛðRASì¶^ÉÀè·é±ÆÅAFOLFOX{BevacizumabæèàLøÅ éÂ\«ª¦´³ê½B
E20050181±
@20050181±ÅÍAñ¡Ãáɨ¯éFOLFIRIÉηéPanitumumabÌãæ¹øʪ¢³ê½B
@
KRASì¶^ÅÍAOSɨ¢ÄÍLÓ·ðFßÈ©Á½àÌÌiFOLFIRI{Panitumumab vs. FOLFIRI=14.5 vs. 12.5, HR=0.85, p=0.12jAåv]¿ÚÌ1ÂÅ éPFSieX5.9 vs. 3.9, HR=0.73, p=0.004j¨æÑtø¦ieX35% vs. 10%, p<0.001jɨ¢ÄAPanitumumab¹pQªLÓÉFOLFIRIPÆQðãñÁ½BêûAKRASÏÙ^ÅÍAPFSAOSAtø¦Ì¢¸êɨ¢ÄàAPanitumumab¹pÉæéøÊÌãæ¹ÍFßçêÈ©Á½57)B
@
{ÊæèAKRASì¶^Ìñ¡Ãɨ¯éPanitumumabÌLø«ª¦³ê½B
@ȨA2012NÄÕ°îáwïNWïɨ¢Ä{±ÌÅIo^30ãÌðͪñ³ê½BKRASì¶^ɨ¯éPFSÍFOLFIRI{PanitumumabQ6.7AFOLFIRIPÆQ4.9iHR=0.82, 95% CI: 0.69-0.97, p=0.023jAtø¦ÍeX36.0% vs. 9.8%iIbYä5.50, 95% CI: 3.32-8.87, p<0.0001jÆAPanitumumab¹pQªLÓÉãñÁ½72)BOSÍeX14.5 vs. 12.5iHR=0.92, 95% CI: 0.78-1.10, p=0.366jÆPanitumumab¹pQÅ·¢XüÉ Á½àÌÌLÓ·ÍFßçêÈ©Á½BGrade 3/4ÌLQÛÉ¢ÄàçáQ ieX37% vs. 2%jâºieX14% vs. 9%jAPanitumumab¹pQŽFßç꽪A·úÏ@Éæé¾ç©ÈLQÛÌÁÍFßÈ©Á½BܽAKRASÏÙ^ɨ¢ÄÍOSAPFSAtø¦Æà¯öxÅ Á½B
ESPIRITT±
@SPIRITT±ÍAKRAS exon 2ì¶^ÅBevacizumabÆL-OHPx[X̹pÃ@ÉsÆÈÁ½ÇáðÎÛÉA2nd-lineɨ¢ÄFOLFIRI{PanitumumabÆFOLFIRI{Bevacizumabðärµ½³ì×»æII±Å éBåv]¿ÚÅ éPFSlÍAPanitumumabQ7.7ABevacizumabQ9.2iHR=1.01, 95% CI: 0.68-1.50jAOSlÍ»ê¼ê18.0A21.4ÅiHR=1.06, 95% CI: 0.75-1.49jAÆàɼQÔÉLÓ·ðFßÈ©Á½90)BȨAtø¦Í»ê¼ê32%A19%ÆAPanitumumabQÅÇDÅ Á½B
@Grade 3/4ÌLQÛÍPanitumumabQ78%ABevacizumabQ65%ÉFßALQÛÉæé¡Ã~ÍA»ê¼ê29%A25ƼQůöxÅ Á½BREGFRRÌòÉæéã¡ÃÍAPanitumumabQ26%ABevacizumabQ54%ARVEGF»ÜÉæéã¡ÃÍ»ê¼ê20%A24%Å Á½B
@PEAK±¯lA{±Í¼àÝèÌÈ¢±Å Á½ªAL-OHP¨æÑBevacizumabsÌKRAS exon 2ì¶^ɨ¢ÄAFOLFIRI + PanitumumabÆFOLFIRI + BevacizumabÌOSͯöxÅ Á½B
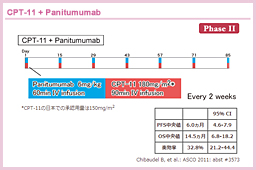
@GERCOR±Ítb»s~Wn»ÜAL-OHPACPT-11ÉsÆÈÁ½KRASì¶^ɨ¯éCPT-11i180mg/m2j{Panitumumabi6mg/kgjÌLø«ð¢µ½PA[æII±Å éBåv]¿ÚÍtø¦ÅAúÒlð30%ÉÝèµ½B»èÉæéKRASâ`q¸ÉÄÏÙðFßÈ©Á½61áªðÍÎÛÆÈèAtø¦Í32.8%i20/61ájAa¨Rg[¦Í68.9%i42/61ájÅ Á½73)BÏ@úÔl16.8Ì_ÅAPFSÍ6.0i95% CI: 4.6-7.9jAOSÍ14.5i95% CI: 6.8-18.2jÅ Á½B
@CPT-11sáÉηéREGFRRÌòÆCPT-11̹pÉ¢ÄÍAæÉBOND±ÅCetuximab¹pÉæéPFSÌ·øʪ¦³êÄ¢½ª52)APanitumumabÉ¢Äà¯lÈʪ¦´³ê½B
@PICCOLO±ÍAtb»s~Wn»ÜÉæé¡Ãðª èACPT-11gpðÌÈ¢ÇáðÎÛÉACPT-11{PanitumumabACPT-11{CiclosporinACPT-11PÆÌ3Qðärµ½æIII±Å éB2008N6ÉvgR[ªüù³êAKRASì¶^ɨ¯éCPT-11PÆÆCPT-11{Panitumumabðär·éÝèÉÏXÆÈÁ½B
@KRASì¶^460áªo^³êAåv]¿ÚÅ éOSÍCPT-11{PanitumumabQ10.4ACPT-11PÆQ10.5Å èALÓ·ðFßÈ©Á½iHR=0.91, 95% CI: 0.73-1.14, p=0.44j74)BI]¿ÚÅ éPFSÍAeX5.5 vs. 4.7iHR=0.78, 95% CI: 0.64-0.95, p=0.01jAtø¦ÍeX34% vs. 12%ip<0.0001jÆA¢¸êàPanitumumab¹pQÅLÓÉÇDÅ Á½B
@ܽA{±ÅÍ ç©¶ßKRASÈOÌBRAFAPIK3CAANRASâ`qÌðͪ\è³êÄ¢½B·×ÄÌâ`qªì¶^ÌÇáɨ¢ÄPanitumumab¹pÍPFS̷𦵽ªiHR=0.70, p=0.01jAOSÅÍLÓ·ðFßÈ©Á½iHR=0.86, p=0.32jBêûA¢¸ê©Ìâ`qÉÏÙðLµÄ¢éÇáÅÍCPT-11PÆQÅOSªLÓÉÇDÅ èiHR=2.03, p<0.01jAùñƯlÉBRAFAPIK3CAANRASâ`qàPanitumumabgpɨ¯éoCI}[J[ÆÈéÂ\«ª¦´³ê½B
@AfliberceptÍ2íÌVEGFóeÌiVEGFR-1AVEGFR-2jÌ×EOhCÌêðqgÆuOuG1iIgG1jÌFcÌæÉZ³¹½g·¦Z`¿Å éBìp@ƵÄÍAVEGFóeÌKhÅ éVEGF-AAVEGF-B¨æÑÙÕBöqiPlGFjɵÄîáɨ¯éÇV¶Æǧ߫ðjQ·é±Æªl¦çêÄ¢éB
@VELOUR±ÅÍAL-OHPx[XÌñ¡ÃÉsÆÈÁ½ÇáðÎÛÉAFOLFIRIÉηéAfliberceptÌãæ¹øʪ¢³ê½Båv]¿ÚÌOSÍFOLFIRI{AfliberceptQ13.5AFOLFIRIPÆQ12.1iHR=0.82, 95.34% CI: 0.71-0.94, p=0.0032jA]¿ÚÌPFSÍeX6.9 vs. 4.7iHR=0.76, 99.99% CI: 0.58-1.10, p=0.00007jAtø¦ÍeX19.8% vs. 11.1%ip=0.0001jÆ¢¸êàAfliberceptÉæéãæ¹øʪFßçê½75)B
@ÈãÌÊæèA2012N8ÉÄFDAųF³êANCCNKChCi2013NÅversion1jɨ¢Ä৳êé¡Ã@Ì1ÂƵÄfÚ³êÄ¢éi2012N12_Å{M¢³Fj67)B
@ܽAOÉ\è³êÄ¢½O¡Ãɨ¯éBevacizumab^ÌL³ÉæéAfliberceptÌøÊÉ¢ÄàðͪsíêABevacizumabgpðÌ éÇáɨ¯éOSÍeX12.5 vs. 11.7iHR=0.862, 95.34% CI: 0.673-1.104jABevacizumabgpðÌÈ¢Çáɨ¢ÄÍAeX13.9 vs. 12.4iHR=0.788, 95.34% CI: 0.699-0.927jÅ èABevacizumab^ðÌL³ÉæéðÝìpÍFßÈ©Á½ip=0.57jB
@Grade 3/4ÌLQÛͺAÓ´AûàA´õA³A ÉAD
¸A`AªAflibercept¹pQŽFßçêALQÛɺ¤¡Ã~áÍAflibercept¹pQÅ26.6%AFOLFIRIPÆQÅ12.1%ÆAflibercept¹pQŽ¢XüÉ Á½B
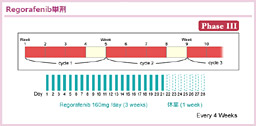
@RegorafenibÍîáÌBE¶¶AÇV¶ÉÖ^·éVEGFRAKITARETAFGFRAPDGFRÈÇðWIÆ·éoû}`Li[[jQÜÅ éB
@CORRECT±ÅÍAW»wÃ@A·Èí¿tb»s~Wn»ÜAL-OHPACPT-11ABevacizumabAKRASì¶^ɨ¯éREGFRRÌòÉsÆÈÁ½ÇáðÎÛÉARegorafenibÌLp«ª¢³ê½Båv]¿ÚÅ éOSÍAOÉKè³ê½ÔðÍÌ_ÅARegorafenibQ6.4AplaceboQ5.0iHR=0.77, 95% CI: 0.64-0.94, p=0.0052jÆRegorafenibQªLÓÉãñèALø~ÆÈÁ½76)BI]¿ÚÅ éPFSɨ¢ÄàeX1.9 vs. 1.7iHR=0.525, 95% CI: 0.42-0.58, p<0.000001jÆRegorafenibQÅLÓÉÇDÅ Á½BLQÛÍARegorafenibQÅgrade 3ÈãÌçáQAÌ@\áQAæJª½Fßçê½B
@ܽAKRASÏÙÌL³ÉæéTuO[vðÍàñ³êAKRASì¶^ɨ¯éOSÍeX7.3 vs. 5.0iHR=0.653, 95% CI: 0.476-0.895jAKRASÏÙ^ɨ¯éOSÍeX6.2 vs. 5.1iHR=0.867, 95% CI: 0.670-1.123jÆKRASÏÙÌL³É©©íç¸RegorafenibQÅÇDÅ Á½B
@ÈãÌÊæèA2012N9ÉÄFDAųFBNCCNKChCi2013NÅversion1jɨ¢Ä৳êé¡Ã@Ì1ÂƵÄfÚ³ê½67)BܽA{MÅà2013N325úɳFðæ¾µ½B
@ȨAESMO 2012ÅͶ¶úÔÌup dateªñ³êA»ê¼ê̱QÌOSÍ6.4 vs. 5.0iHR=0.79, 95% CI: 0.66-0.94, p=0.0038) ÆA2012NÄÕ°îáwïNWïÅÌÔñƯlÌÊÅ Á½77jB
GI cancer-net
Á»íà¡ÃÌLê